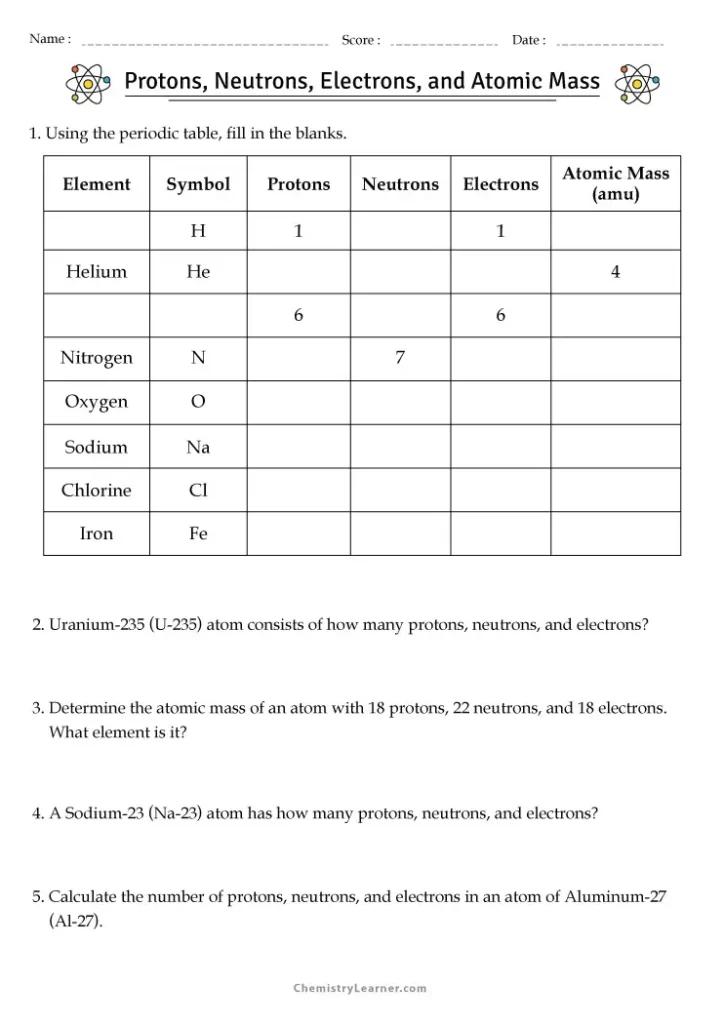
Protons, Neutrons, and Electrons Practice Worksheets
What are Protons, Neutrons, and Electrons?
Protons, neutrons, and electrons are the three main particles that make up an atom. They each have different properties and play important roles in determining the characteristics of an element.
Protons
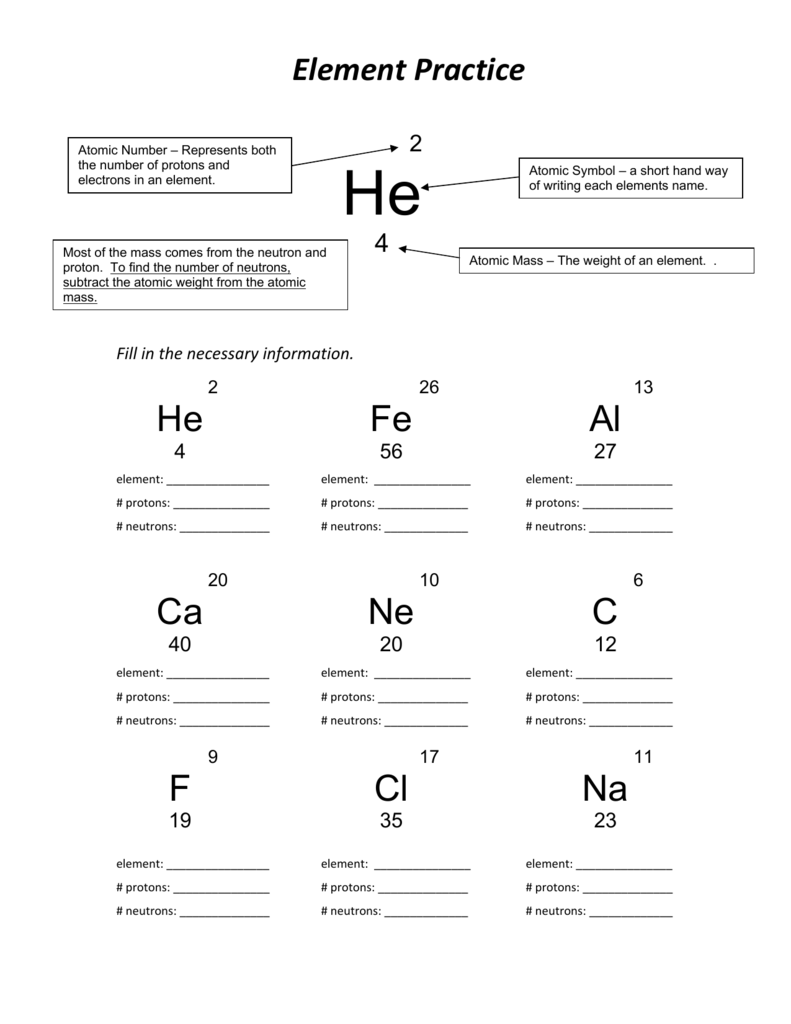
Protons are positively charged particles found in the nucleus of an atom. They have a mass of approximately 1 atomic mass unit (amu). The number of protons in an atom determines its atomic number and identifies the element. For example, hydrogen has 1 proton, while carbon has 6 protons.
Neutrons
Neutrons are particles found in the nucleus of an atom that have no charge, hence the name “neutral.” They have a mass similar to protons, approximately 1 amu. The number of neutrons can vary within the same element, resulting in different isotopes. Isotopes have the same number of protons but different numbers of neutrons.
Electrons
Electrons are negatively charged particles that orbit the nucleus of an atom in energy levels or shells. They have a negligible mass compared to protons and neutrons. The number of electrons in an atom is equal to the number of protons, ensuring overall electrical neutrality. Electrons are involved in chemical reactions and determine the atom’s reactivity.
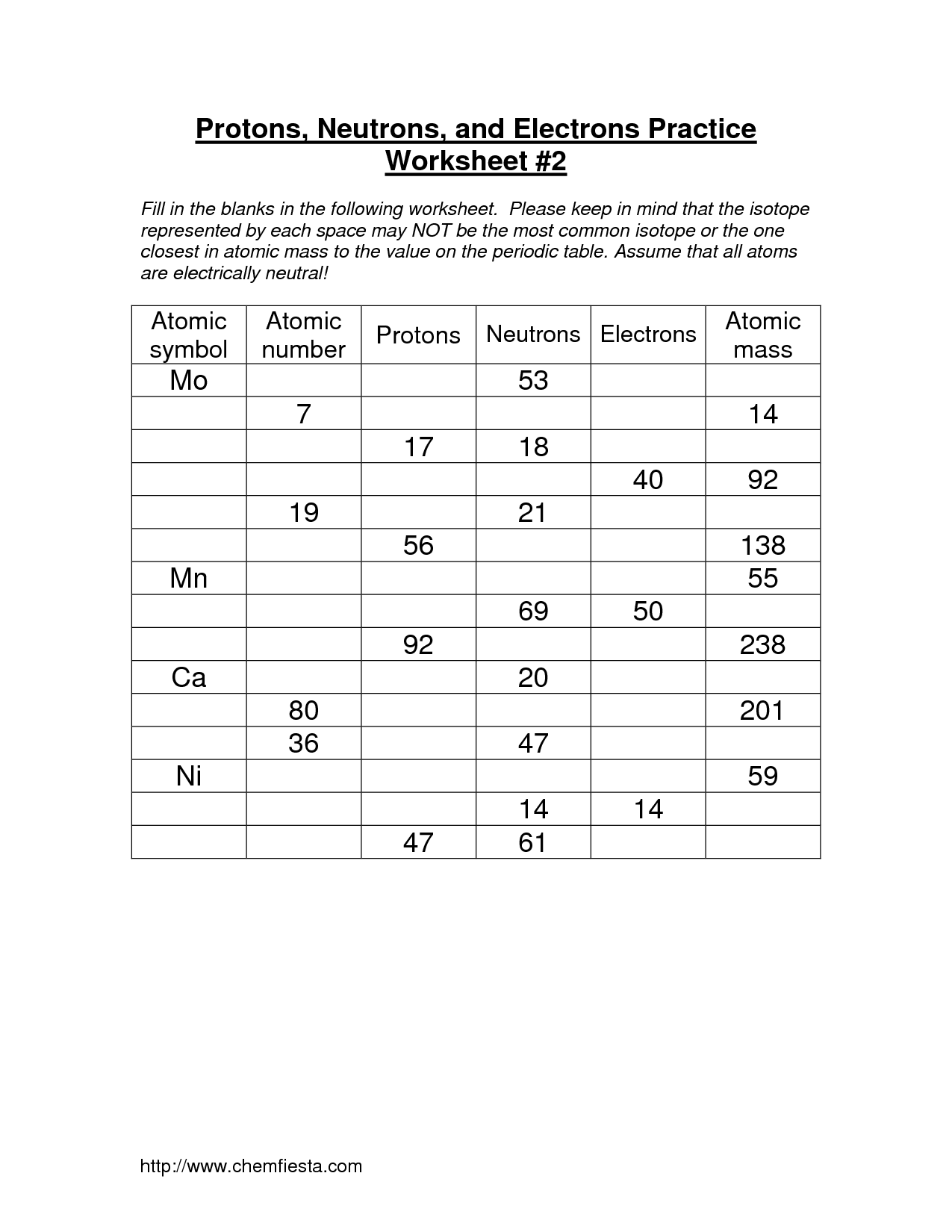
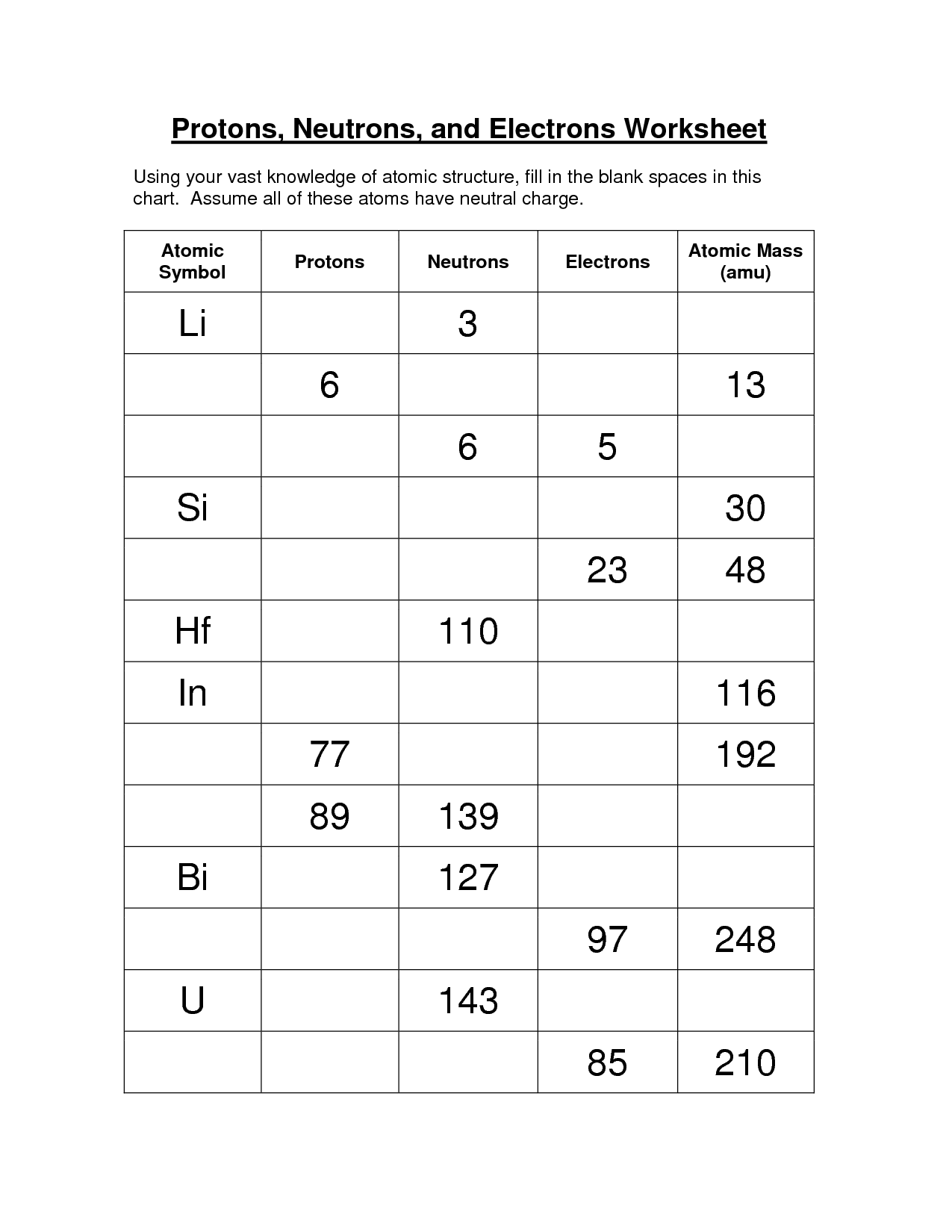
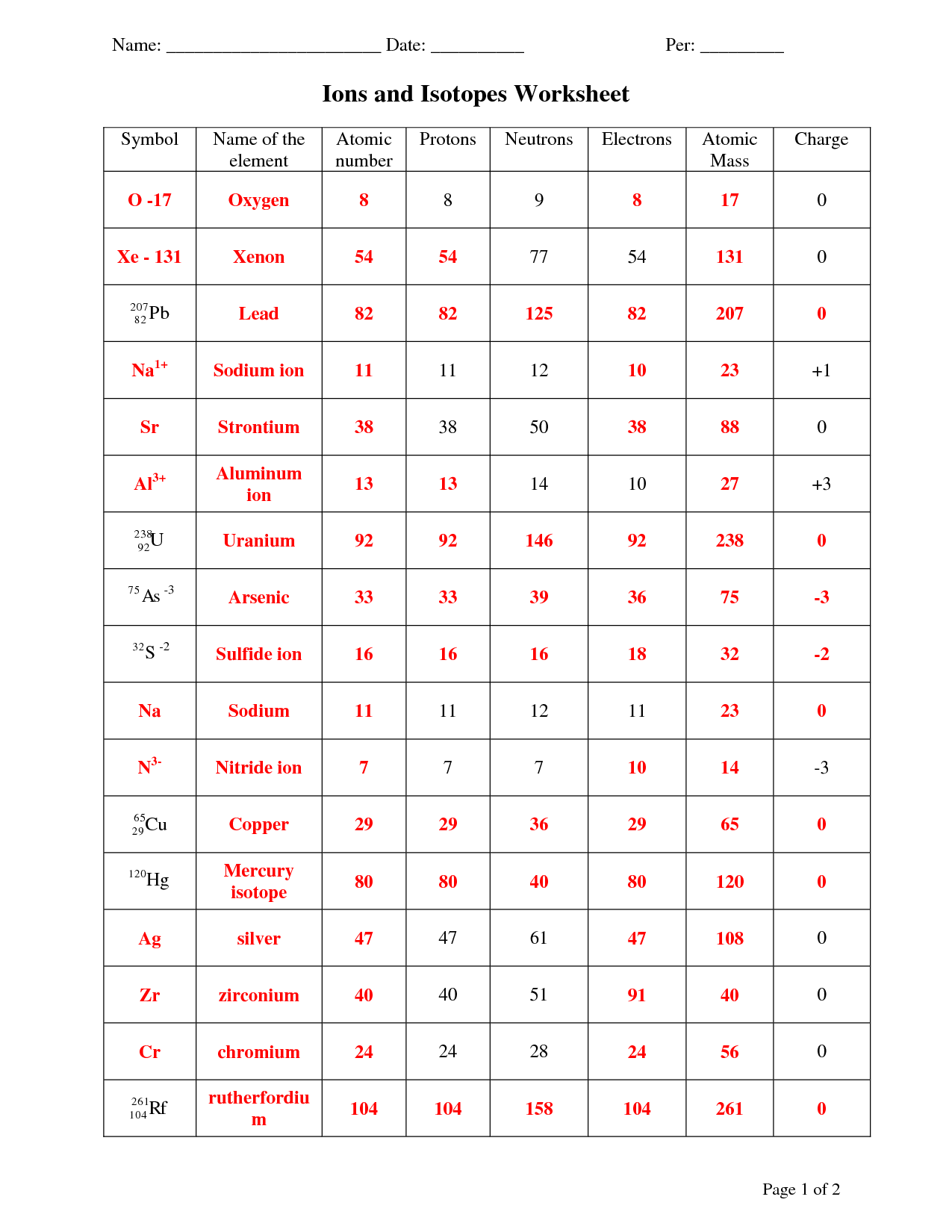
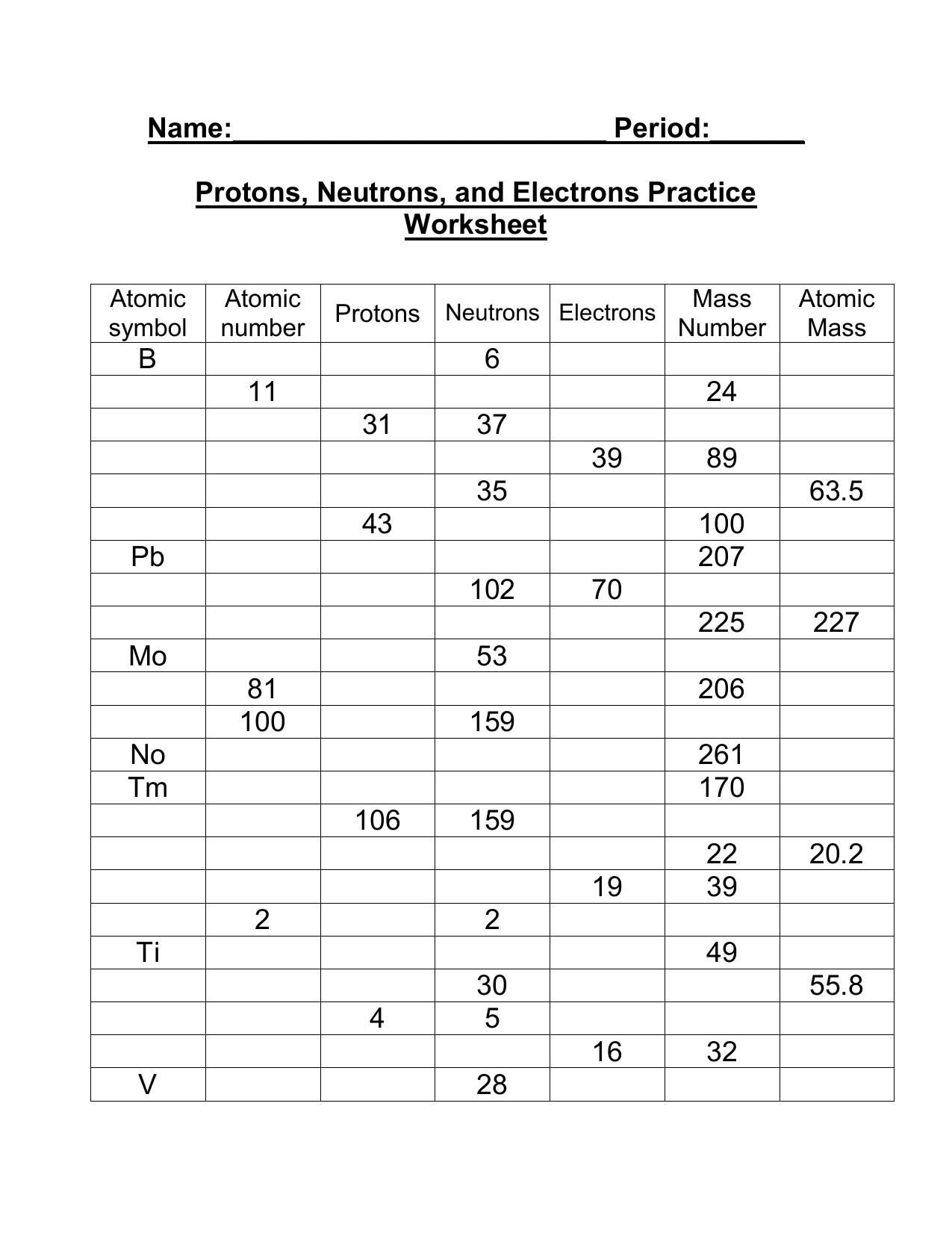
Practice Worksheets
To reinforce your understanding of protons, neutrons, and electrons, practice worksheets can be a helpful tool. These worksheets typically provide questions and exercises that test your knowledge of atomic structure and the properties of these particles.
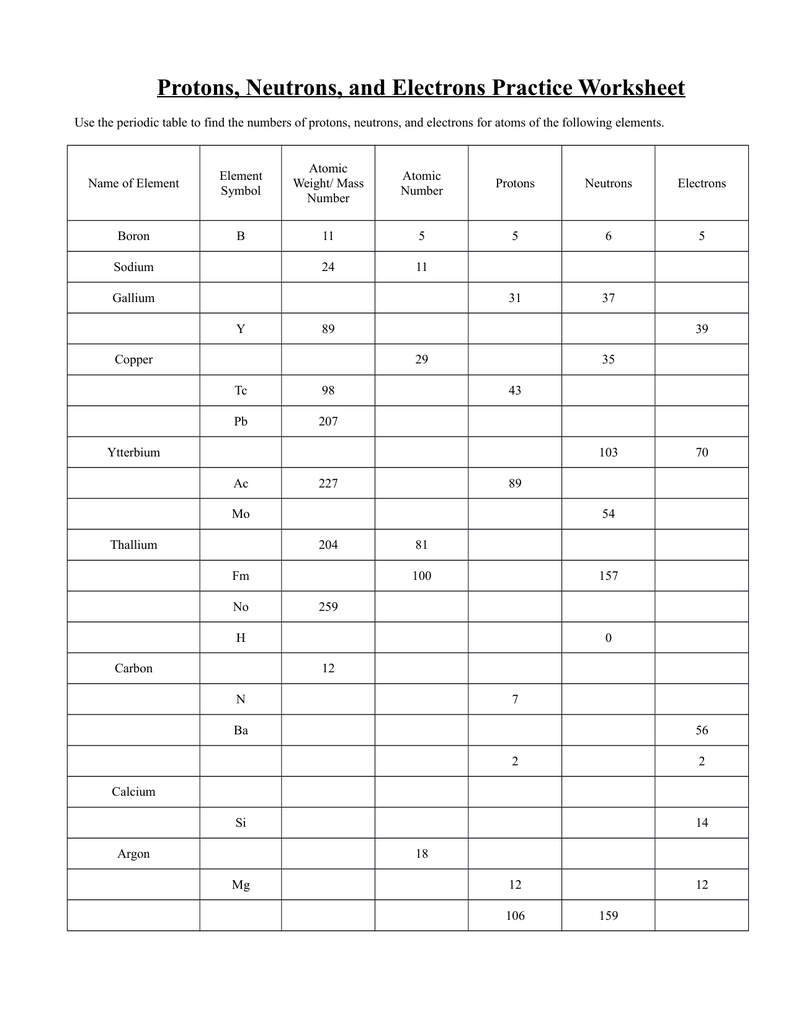
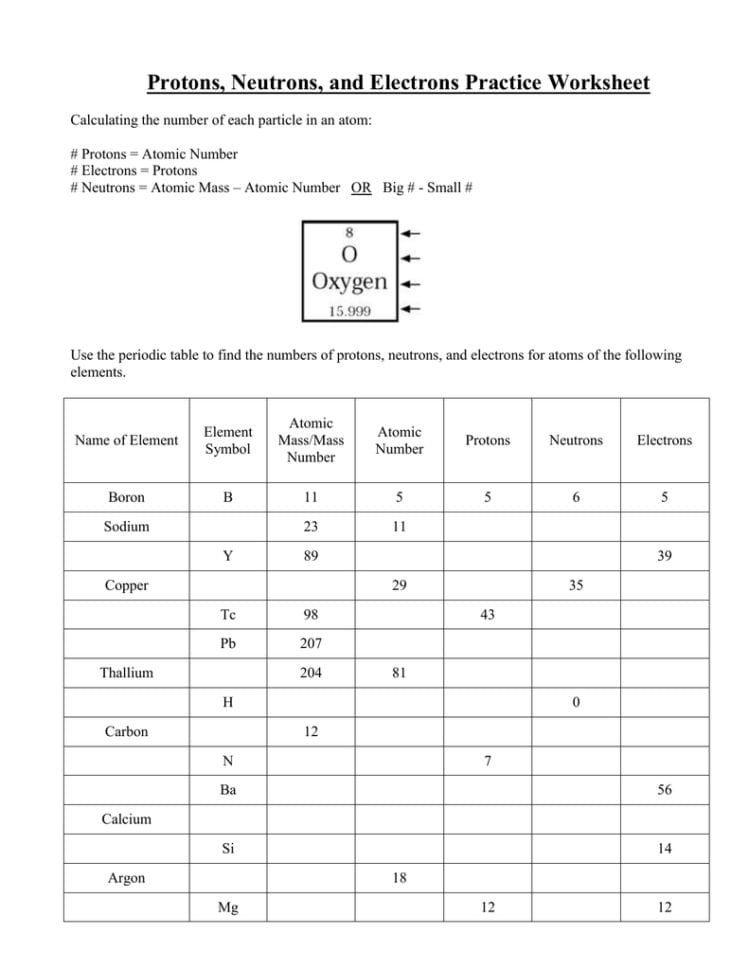
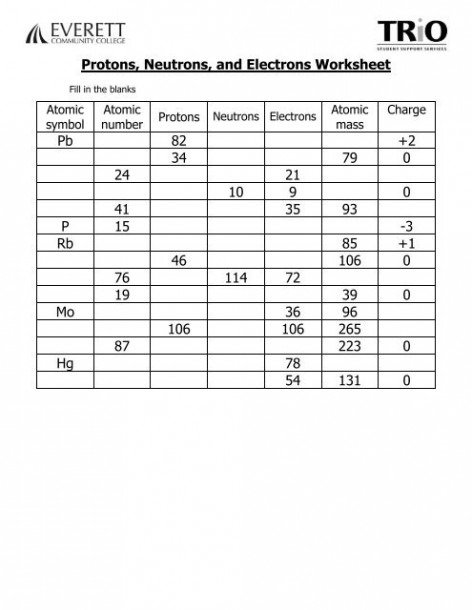
You may be asked to identify the number of protons, neutrons, and electrons in a given element or isotope. This helps you grasp the concept of atomic number, mass number, and how they relate to the composition of an atom.
Additionally, practice worksheets can include questions about the distribution of electrons in different energy levels or the arrangement of electrons in electron configurations. This allows you to practice applying the rules for filling electron shells.
By completing these worksheets, you can develop a solid understanding of the fundamental building blocks of matter and their properties. Regular practice will enhance your ability to analyze and predict the behavior of atoms and elements.
Remember to check your answers and seek clarification if you encounter any difficulties. Practice worksheets serve as a valuable tool for reinforcing your knowledge and preparing for exams or assessments.
Conclusion
Protons, neutrons, and electrons are essential components of an atom. Understanding their properties and roles is crucial in chemistry and physics. Practice worksheets provide an interactive way to reinforce your understanding and improve your skills in dealing with atomic structures and characteristics. Regular practice will help you become more confident in your knowledge and abilities in this subject area.
Protons Neutrons And Electrons Practice Worksheet
Protons Neutrons And Electrons Practice Sheet
Protons Neutrons Electrons Practice Worksheets
Protons Electrons And Neutrons Worksheet
Protons Neutrons And Electrons Practice Worksheets With Answers
Atomic Number Worksheet
Free Printable Protons Neutrons And Electrons Practice Worksheets